It's World Digestive
Health Day
Patients suffering from acute gastroenteritis require appropriate intervention and treatment from physicians. Many gastrointestinal illnesses have similar or overlapping symptoms1, making it difficult to define a cause and adding complications to diagnosis.
For patients with Inflammatory Bowel Disease (IBD), the similar clinical presentations and laboratory findings in IBD relapse and enteric infections pose barrier to accurate diagnosis and treatment2. It is clinically imperative to differentiate an enteric infection from a flare-up to determine if the symptoms are from an infectious cause.
From our most advanced syndromic approach to trusted traditional methods, we offer a complete diagnostic solution to identify pathogens causing gastrointestinal diseases, making it easier to treat patients and improve outcomes.

OUR SOLUTIONS
1. Syndromic Approach
BIOFIRE® FILMARRAY® Gastrointestinal Panel
 The BIOFIRE FILMARRAY Gastrointestinal (GI) Panel is a PCR-based qualitative multiplexed in vitro diagnostic test. Using the syndromic testing approach, the BIOFIRE GI Panel identifies simultaneously 22 gastrointestinal pathogens (bacteria, viruses, and parasites) in about 1 hour, from one patient stool sample. The BIOFIRE FILMARRAY GI Panel can be run directly on-site at your clinic, on the BIOFIRE FILMARRAY TORCH and 2.0 systems.
The BIOFIRE FILMARRAY Gastrointestinal (GI) Panel is a PCR-based qualitative multiplexed in vitro diagnostic test. Using the syndromic testing approach, the BIOFIRE GI Panel identifies simultaneously 22 gastrointestinal pathogens (bacteria, viruses, and parasites) in about 1 hour, from one patient stool sample. The BIOFIRE FILMARRAY GI Panel can be run directly on-site at your clinic, on the BIOFIRE FILMARRAY TORCH and 2.0 systems.
Compared to traditional stool testing, the BIOFIRE GI Panel
Reduces time to result by 84%5
Increases diagnostic yield by more than 30%6 helping you to find the cause of GI illness more efficiently
Specifically, for IBD patients, during an exacerbation of symptoms, the BIOFIRE GI Panel has been shown to:
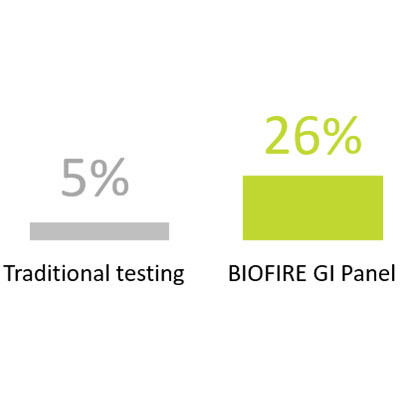
Have a higher rate of pathogen detection compared to traditional testing (26% vs 5%) in outpatients7
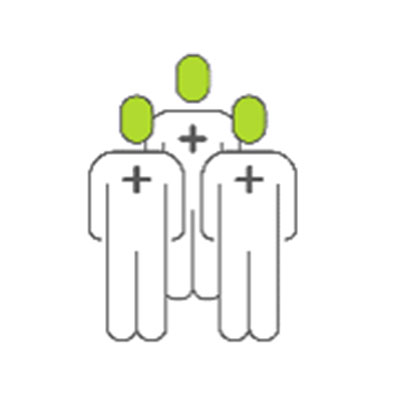
Detect enteric infection in 26.8% of tests, C. difficile being the most common infection2

Detect a non-C. difficile infection in 13,8% of tests2
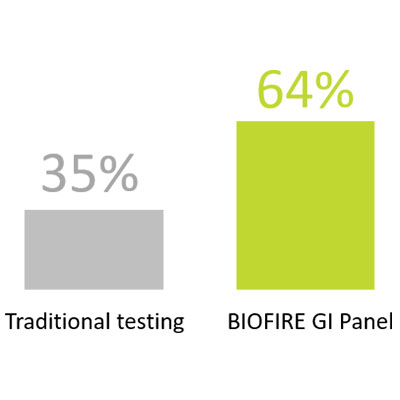
Have a lower rate of IBD treatment modification compared to traditional testing (35% vs 64%)8
In addition, the BIOFIRE GI Panel is suggested to be used as a first diagnostic tool in patients suffering from IBD to target the subpopulations of patients needing diagnostic endoscopy9. With advanced molecular tests, physicians can better direct treatment strategies for IBD patients and more accurately pinpoint the cause of Gastrointestinal illness.
Learn more about our complete syndromic testing solutions or download the BIOFIRE® FILMARRAY® Gastrointestinal Panel CIM Pamphlet
Our complete syndromic testing solutions
References:
- Riddle MS et al. Am J Gastroenterol. 2016; 111:602-622.
- Axelrad JE, et al. Inflamm Bowel Dis. 2017 Jun; 23(6):1034-1039.
- Shane AL et al. Clin Infect Dis. 2017;65(12): e45-e80.
- Overall performance based on prospective clinical study for the BioFire® FilmArray® Gastrointestinal Panel, data on file, BioFire Diagnostics.
- Beal S, et al. JCM. Jan 2018, 56 (1): JCM. 01457-17.
- Meyer j, et al. Scand J Gastroenterol. 2020; Dec;55(12):1405-1410.
- Hong S, et al. Inflamm Bowel Dis. 2021 Oct; 27(10):1634-1640.
- Ahmad W, et al. Dig Dis Sci. 2019 Feb; 64(2): 382–390.
- Meyer, J., J et al . Sci Rep 2022 12(1): 9730
2. Traditional Approach

CHROMID® IDENTIFICATION
Chromogenic Media for Organism Isolation and Identification. GI-related media include:
- CHROMID® Salmonella Elite ref 412108
- CHROMID® Vibrio ref 43762
- CHROMID® Candida ref 43639
- CHROMID® Candida ref 43631
- Biplate CHROMID® Candida/Sabouraud ref 43464
- CHROMID® C Difficile ref 43871

BIOMERIEUX EPISEQ®
Evidence of the role of microbiota has grown in recent years. Today, thanks to the high throughput of Next Generation Sequencing1,2, you can study the dynamics of a patient’s microbiome and investigate its impact on patient health.
EPISEQ® 16S can accelerate the discovery of biomarkers with a very easy to use Software as a Service solution for data analysis with all statistical tools on board.